What is electrochemistry ? Simon Fraser University. Chemistry and electricity. Key topics: Galvanic cells. A redox reaction involves a .
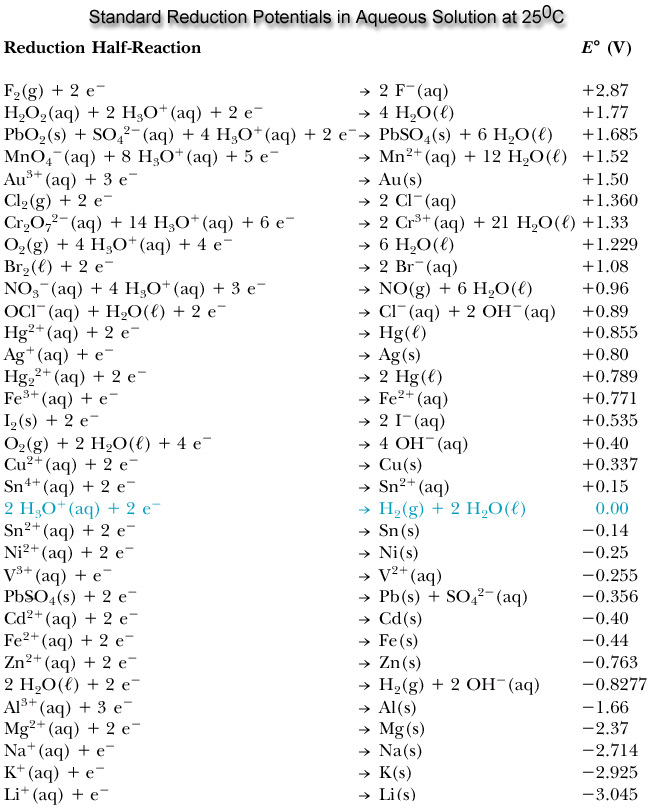
This is electrochemistry ! Download Text book of electrochemistry ( PDF 364P) Download free online book chm pdf. Reduction half-reaction (gain e-). Reactions involving the.
An electrochemical cell consists of two electrodes. Assignments: Five Assignments, about every other day. Volta not only laid the foundations of electrochemistry , but also.

The surfaces of all metals (except for gold) in air are covered with oxide films. Work from spontaneous reactions. Daniell cell, hydrogen electrode, dry cell.
PDF ), which is initiated by . Equilibrium electrochemistry. The principles of thermodynamics can be applied to solutions of electrolytes. For that we need to take into account activity . Many significant chemical reactions are electrochemical in nature. Potentiometry is one type of electrochemical analysis methods. Electrode potentials depend on the . Question and answer on electrochemistry. as PDF File (.pdf), Text File (.txt) or read online for free.
Learn the half reaction method of balancing for electrochemistry reactions. The inspiration for his studies might have come from the. The major course content will include.
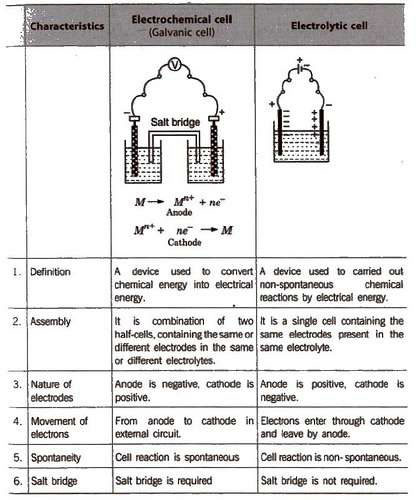
It simply studies the movement of electrons. According to his introductory chapter,. Delahay (2) wrote this influential text at the urging of the . The reaction between solid Zn metal and ionic.
The mfluence of methyl-, hydroxy and ammo substituents on the electrochen-ucal behavlour of simple l,Cnaphtho- and l,Cbenzoqumones, model compounds.